Market Overview:
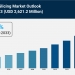
The Hernia Mesh Devices Market is experiencing steady expansion, driven by Rising Preference for Laparoscopic Procedures, Expanding Adoption of Robotic-Assisted Surgery, and Increasing Focus on Patient-Specific Solutions. According to IMARC Group's latest research publication, "Hernia Mesh Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global hernia mesh devices market size reached USD 2.06 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 2.63 Billion by 2033, exhibiting a growth rate (CAGR) of 2.76% during 2025-2033
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Download a sample PDF of this report: https://www.imarcgroup.com/hernia-mesh-devices-market/requestsample
Our Report Includes:
- Market Dynamics
- Market Trends and Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Growth Factors in the Hernia Mesh Devices Market Industry:
- Rising Preference for Laparoscopic Procedures
Laparoscopic hernia repair usually involves the use of a camera and small incisions to guide the placement of the mesh. This, in turn, results in quicker recovery times, less postoperative pain, and reduced hospital stays compared to traditional open surgery. In April 2024, one of the medical technology companies, TELA Bio, announced the commercial launch of OviTex inguinal hernia repair (IHR) reinforced tissue matrix, a product tailored for laparoscopic and robotic-assisted procedures, in the U.S. It offers flexibility for surgeons based on patient needs and procedural preferences. The shift toward minimally invasive surgical techniques has led to significant growth in demand for specialized mesh devices designed for laparoscopic applications, as they provide better patient outcomes and enable faster return to normal activities.
- Expanding Adoption of Robotic-Assisted Surgery
The inflating need for improving the accuracy of mesh placement, more complex maneuvers, and minimizing the risk of complications is catalyzing the market. Robotic-assisted surgery also offers benefits, such as reduced blood loss, smaller incisions, and quicker recovery times, for patients. In May 2024, Medtronic initiated additional clinical studies of its Hugo robotic-assisted surgery system to expand its indications to hernia and gynecology. This is bolstering the hernia mesh devices market. The precision and control offered by robotic systems enable surgeons to perform complex hernia repairs with enhanced visualization and dexterity, leading to improved surgical outcomes and reduced complications. This technological advancement has positioned robotic-assisted hernia repair as a premium treatment option in leading healthcare facilities globally.
- Increasing Focus on Patient-Specific Solutions
Numerous advancements in imaging technologies and computer modeling, which enable precise preoperative planning and customized mesh designs, are catalyzing the market. Patient-specific solutions aim to minimize complications, enhance the success rates of hernia repairs, and improve overall patient satisfaction. In January 2024, Absolutions received an FDA breakthrough designation for an abdominal wall closure device to reduce the risk of hernia by distributing suture tension over a large area of tissue. The personalization of hernia mesh devices based on individual patient anatomy and specific hernia characteristics represents a paradigm shift in surgical care, allowing for tailored treatment approaches that address unique patient needs and optimize long-term outcomes.
Key Trends in the Hernia Mesh Devices Market:
- Increasing Number of Hospitals, Clinics, and Ambulatory Surgical Centers
The rising occurrence of hernia, on account of diarrhea or constipation, obesity, persistent coughing, poor nutrition, smoking, and overexertion, is stimulating the market. The rising adoption of hernia mesh devices in hospitals, clinics, and ambulatory surgical centers, as they enhance patient outcomes by minimizing operative time, is primarily driving the global hernia mesh devices market growth. These facilities are increasingly equipped with advanced surgical technologies and skilled personnel capable of performing complex hernia repairs. The expansion of ambulatory surgical centers, in particular, has democratized access to hernia repair procedures, offering patients convenient, cost-effective alternatives to traditional hospital-based surgery while maintaining high standards of care and safety.
- Introduction of Articulating Fixation Devices
The introduction of articulating fixation devices that offer surgeons better access to weak spots within the abdominal wall and enable them to place the mesh at the desired body site securely is acting as a significant growth-inducing factor. These innovative devices provide enhanced maneuverability and precision during mesh placement, allowing surgeons to navigate anatomically challenging areas with greater ease. In January 2024, TELA Bio Inc., a commercial-stage medical technology company, introduced LIQUIFIX precision and LIQUIFIX FIX8 laparoscopic open hernia mesh fixation devices in the U.S. Both devices are the only FDA-approved devices that affix mesh and approximate peritoneal tissue with liquid anchors, representing a breakthrough in fixation technology that improves procedural efficiency and patient outcomes.
- Advancements in Biocompatible Materials
The growing focus on advancing biocompatible materials is expected to fuel the market in the coming years. Manufacturers are developing mesh devices using materials that promote better tissue integration, reduce inflammatory responses, and minimize the risk of complications such as adhesion formation and infection. For example, Medtronic introduced the Parietex Hybrid Mesh, which combines non-absorbable and absorbable components to enhance biocompatibility and tissue integration. Similarly, Bard unveiled the Ventralight ST Mesh, featuring a lightweight, large-pore design with a bioresorbable coating that promotes better tissue integration. These material innovations address long-standing challenges in hernia repair and represent the future direction of mesh device development.
Leading Companies Operating in the Global Hernia Mesh Devices Market Industry:
- B. Braun SE
- Becton, Dickinson and Company
- Cook Medical
- Deep Blue Medical Advances, Inc.
- Dipromed Srl
- Herniamesh S.r.l.
- Johnson & Johnson
- Medtronic
- Meril
- Novus Scientific AB
- Stryker Corporation
- W. L. Gore & Associates, Inc.
Hernia Mesh Devices Market Report Segmentation:
Breakup by Hernia Type:
- Inguinal Hernia
- Incisional Hernia
- Femoral Hernia
- Others
Inguinal hernia exhibited a clear dominance in the market due to its high prevalence as the most common type of hernia, accounting for the majority of hernia repair procedures worldwide. An inguinal hernia occurs when a portion of the intestine protrudes through a weakened spot in the abdominal muscles. The significant incidence rate, particularly among adult males, combined with the availability of well-established surgical techniques and mesh products specifically designed for inguinal hernia repair, has positioned this segment as the market leader.
Breakup by Mesh:
- Biologic Mesh
- Synthetic Mesh
Synthetic mesh exhibited a clear dominance in the market due to their cost-effectiveness, durability, widespread availability, proven clinical track record, and suitability for a wide range of hernia repair procedures. Synthetic mesh devices represent medical implants made from materials like polypropylene, designed to provide support to weakened or damaged tissue. Their mechanical strength, predictable performance characteristics, and lower cost compared to biologic alternatives have made them the preferred choice for the majority of hernia repairs globally.
Breakup by End User:
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
Hospitals exhibited a clear dominance in the market due to their comprehensive surgical infrastructure, availability of advanced medical equipment, skilled surgical teams, ability to handle complex cases, and established patient referral systems. Hernia mesh devices are medical implants used in hospitals to provide additional support to weakened or damaged tissue. Hospitals remain the primary setting for hernia repair procedures, particularly for complex cases requiring specialized care, extended monitoring, and access to multidisciplinary medical expertise.
Breakup by Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America dominated the global market due to advancements in surgical techniques, growing incidences of hernias, a strong focus on improving patient outcomes, high healthcare expenditure, well-established healthcare infrastructure, increasing demand for robotic hernia surgeries, and robust reimbursement policies. The region benefits from early adoption of innovative surgical technologies, extensive clinical research activities, and a large patient population seeking minimally invasive treatment options. In January 2024, TELA Bio Inc. introduced LIQUIFIX precision and LIQUIFIX FIX8 laparoscopic open hernia mesh fixation devices in the U.S., both being the only FDA-approved devices that affix mesh and approximate peritoneal tissue with liquid anchors, demonstrating the region's leadership in medical device innovation.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel NoD) +91 120 433 0800
United States: +1-201-971-6302









Comments (0)